COVID-19 IgG/IGM Rapid Test Cassette by Healgen (Orient Gene) – 25 tests per box – buyer subject to approval
$495.00
ALL ORDERS MUST SHIP TO AN APPROVED AND VERIFIED MEDICAL CENTER
***these do not ship immediately – our team will communicate approximate ETA once order has been approved***
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma)
This Rapid Test Kit facilitates patient treatment decisions quickly because of the fast 2 – 10 minutes results. This product is simple, time-saving procedure that contains all the necessary reagents. COVID-19 Rapid Test Kit is also high sensitive and specific.
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM antibodies to 2019 Novel Coronavirusi in human whole blood, serum or plasma. This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) must be confirmed with alternative testing method(s) and clinical findings.
- Description
- Reviews (0)
Description
ALL ORDERS MUST SHIP TO AN APPROVED AND VERIFIED MEDICAL CENTER
***these do not ship immediately – our team will communicate approximate ETA once order has been approved***
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma)
This Rapid Test Kit facilitates patient treatment decisions quickly because of the fast 2 – 10 minutes results. This product is simple, time-saving procedure that contains all the necessary reagents. COVID-19 Rapid Test Kit is also high sensitive and specific.
COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM antibodies to 2019 Novel Coronavirusi in human whole blood, serum or plasma. This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) must be confirmed with alternative testing method(s) and clinical findings.
Procedure & Explanation
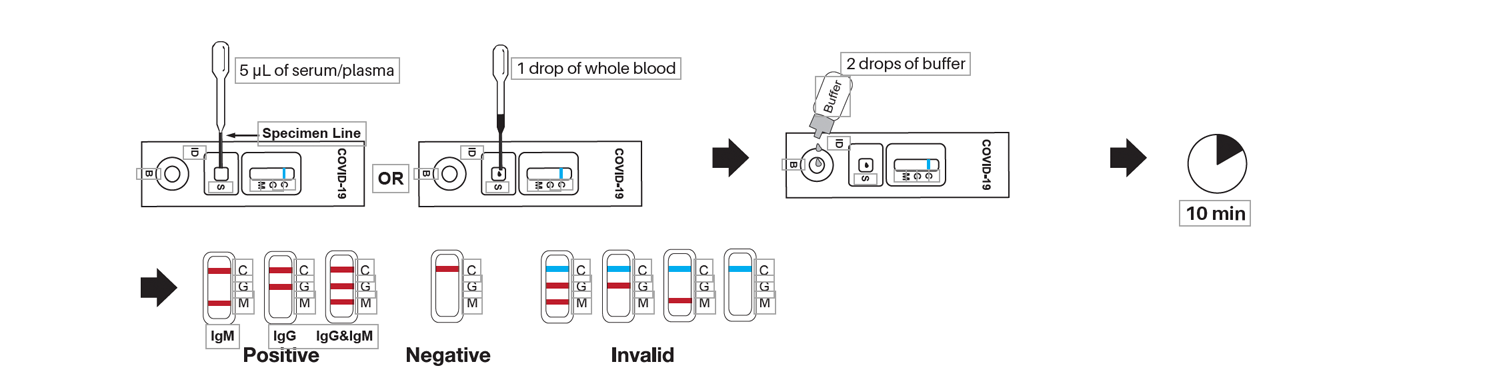
Usage Steps

Package Insert


FDA Submission Number: EUA200056

Disclosure:
The test has not been reviewed by the FDA and results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.





Reviews
There are no reviews yet.